
# 热门搜索 #
华人的骄傲:三十分钟无创癌症精准诊断
{{detail.short_name}} {{detail.main_page}}
{{detail.description}} {{detail.round_name}} {{detail.state_name}}
 提供支持
提供支持 肺癌是除皮肤癌以外的人体第二大常见癌症,也是迄今为止,引发死亡率最高的癌症。自2008年开始,肺癌已取代肝癌成为中国头号癌症杀手。在过去的三十年里,中国人肺癌死亡人数增加了464.85%。非小细胞肺癌(NSCLC)占所有肺癌的85%。鳞状细胞癌、腺癌和大细胞癌都是非小细胞肺癌的子型。
肺癌是除皮肤癌以外的人体第二大常见癌症,也是迄今为止,引发死亡率最高的癌症。自2008年开始,肺癌已取代肝癌成为中国头号癌症杀手。在过去的三十年里,中国人肺癌死亡人数增加了464.85%。非小细胞肺癌(NSCLC)占所有肺癌的85%。鳞状细胞癌、腺癌和大细胞癌都是非小细胞肺癌的子型。
大部分医生认为,预防和早期监测肺癌是与肺癌抗争的最好方式。此前, 医生会向你推荐三种不同类型的肺癌测试: 影像学检测, 痰细胞学活检和组织活检。然而,这些诊断方法不仅麻烦且费用昂贵。只有当体内发现可疑迹象,且患癌症的概率合理时这些诊断方法才有效。绝大多数患者意识到罹患癌症时,已为时已晚。因此, 在过去几十年肺癌病患的存活率非常低。
自2011年以来,美国临床肿瘤医学会(ASCO)和美国国家综合癌症网络(NCCC)为患者推荐先进的检测表皮生长因子受体(EGFR)法来诊断非小细胞肺癌。简单地说,EGFR疗法是通过如geftinib(吉非替尼)或erlotiniv(厄洛替尼)的EGFR-酪胺酸激酶抑制剂靶向治疗肺癌。虽然这大大提高了生存率, 但同样需要高水平的资源, 大量的专业人员和时间。华人科学家掌握无创基因精准诊断核心技术在这样的背景下,一位来自美国加州大学洛杉矶分校UCLA的研究员廖玮博士开始了他的创业之旅,他创办了一家名为易活生物(Yihuo Bio)的基因诊断公司,聘请美国UCLA教授大卫·王(David Wong)担任首席科学家,目标是开发更为精准,更为便捷,更为经济的基因检测超级工具。 美国时间2016年2月12日,廖玮博士和大卫·王教授在美国促进科学学会AAAS 2016 年会暨全球科学大会上,正式向全世界的科学家及媒体介绍了易活生物的划时代技术EFIRM,立即引发了全球上百家媒体的争相报道,动脉网此前对廖玮博士进入了独家英文专访(英文报道附在中文内容后)。EFIRM使用方便但功能强大,它可以准确诊断出最常见的肺癌亚型,通过一项简单的体液分析判断非小细胞肺癌。这一过程被称为“体液活检”,避免了传统活检对人体造成的损伤。所以新的肺癌诊断方法,其优点是不言自明的。除了具有非损伤性,临床测试表明它可以达到99%以上的高检测准确率。它还被证实了廉价高效。廖玮告诉动脉网,EFIRM可以在不足30分钟内诊断出最常见的肺癌亚型。
美国时间2016年2月12日,廖玮博士和大卫·王教授在美国促进科学学会AAAS 2016 年会暨全球科学大会上,正式向全世界的科学家及媒体介绍了易活生物的划时代技术EFIRM,立即引发了全球上百家媒体的争相报道,动脉网此前对廖玮博士进入了独家英文专访(英文报道附在中文内容后)。EFIRM使用方便但功能强大,它可以准确诊断出最常见的肺癌亚型,通过一项简单的体液分析判断非小细胞肺癌。这一过程被称为“体液活检”,避免了传统活检对人体造成的损伤。所以新的肺癌诊断方法,其优点是不言自明的。除了具有非损伤性,临床测试表明它可以达到99%以上的高检测准确率。它还被证实了廉价高效。廖玮告诉动脉网,EFIRM可以在不足30分钟内诊断出最常见的肺癌亚型。 EFIRM的基本原理,是利用超高灵敏度电化学检测方法特异捕获游离肿瘤基因片段,在不经过样品处理和PCR扩增的情况下,直接读取体液中的基因突变信息,因此被叫做电场诱导释放和测量(EFIRM)。在此技术基础上开发的产品被称之为eLB。廖玮表示,EFIRM的核心技术完全掌握在华人手中,EFIRM的仪器和试剂盒完全由易活生物公司开发完成。
EFIRM的基本原理,是利用超高灵敏度电化学检测方法特异捕获游离肿瘤基因片段,在不经过样品处理和PCR扩增的情况下,直接读取体液中的基因突变信息,因此被叫做电场诱导释放和测量(EFIRM)。在此技术基础上开发的产品被称之为eLB。廖玮表示,EFIRM的核心技术完全掌握在华人手中,EFIRM的仪器和试剂盒完全由易活生物公司开发完成。
易活生物公司总部位于美国, 正在中国进行临床试验和建设生产基地,预计到2016年夏天,工厂就能大批量生产出颠覆性的肿瘤基因检测设备和肺癌检测试剂。2016年6月,公司计划美国芝加哥ASCO会议上发布第一代官方产品并正式进军市场。希望受益更多人群廖玮毕业于北京大学化学学院,也是拥有幸福婚姻的成功男士。回国前他在加州大学洛杉矶分校医学院担任研究员。如他所言,他对公司未来拥有清晰的愿景。他诚恳地说:“能受益这项新技术的人越多越好。” “我们有自己的专利,但我们不介意与有相同愿景的人分享,我们想帮助尽可能多的人。”他告诉动脉网。公司计划将先在中国生产,然后迅速推广到整个国内市场,毕竟中国已成为目前世界上第二大经济体。2017年,公司计划将产品推广到全球其他地区。
该产品意义非凡,正符合了廖博士的愿景。打个比方——他想让这个平台变成新一代基因突变检测的“Apple store”,这个平台是向开发者开放的,他希望在不久的将来能够通过基因突变检测到任何疾病。在这个平台上将出现大量的检测应用试剂盒,每一个试剂盒可以检测一种疾病的基因突变,试剂盒是一次性的。其预期价格是当前诊断方法价格的十分之一。
这个产品相对于它的潜在竞争者有三个明显的优势。首先,其他诊断方法都是基于聚合酶链反应(PCR)的。它有明显的弱点——需要组织活检,因此被认为是侵入性的诊断方法。除此之外,为了检测基因突变,PCR需要预先扩增靶标DNA或RNA序列。其次,其检测的准确性比eLB低。PCR体液活检与组织活检的符合率不到70%, 而eLB可以在不进行PCR扩增的情况下,仅仅检测体液就可以达到99%以上的符合率。并且,这种PCR诊断方法是昂贵的,在中国约花费450美元而且特别耗时间,约在10 — 14天之间。相比而言,EFIRM技术预计将花费50美元左右,且不超过30分钟。
廖玮告诉动脉网,这个新的革命性产品可以用在医院或是中心实验室。对于医院来说,产品必须经过国家监管部门的批准之后方可使用,这个还需要1-2年的时间;对于中心实验室,现在就可以使用eLB进行科学研究类的服务,或者为特定人群及志愿者提供检测服务。(英文原文)Revolutionary Device in Non-Small Cell Lung Cancer (NSCLC) Detection and BeyondLung cancer is the second most common cancer in both men and women (not counting skin cancer) and is by far the leader in cancer deaths among both men and women. In 2008 lung cancer replaced liver cancer as leading cause of deaths in China. The registered lung cancer mortality rate has increased 464.85% in the past three decades in the country of the red dragon. Non-small cell lung cancer (NSCLC) accounts for 85% of overall lung cancers. Squamous cell carcinoma, adenocarcinoma, and large cell carcinoma are all subtypes of NSCLC.Most doctors agree that prevention and early detection are often the best way to battle lung cancer. Not long ago, three different kinds of lung cancer tests could be recommended by your doctor: Imaging testing, Sputum cytology and biopsy. Yet these diagnosis methods are bothersome and costly. Only when there is a reasonable probability of having cancer will these diagnosis methods be utilized, i.e., after the detection of a suspicious agent in your organism. A vast majority of patients do not realize they have developed cancer until it is too late. Consequently, the lung cancer survival rate has remained very small in past decades. Since 2011, however, the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCC) have recommended Epidermal Growth Factor Receptor (EGFR) testing for patients with advanced (NSCLC). Simply said, this recent method treats lung cancer through EGFR, more concretely, via EGFR-tyrosine kinase inhibitors such as geftinib or erlotiniv. Although it has improved the survival rate considerably, the EGFR mutation test does not differ from its predecessors in other aspects and similarly requires a high level of resources, numerous professional staff and is time-consuming.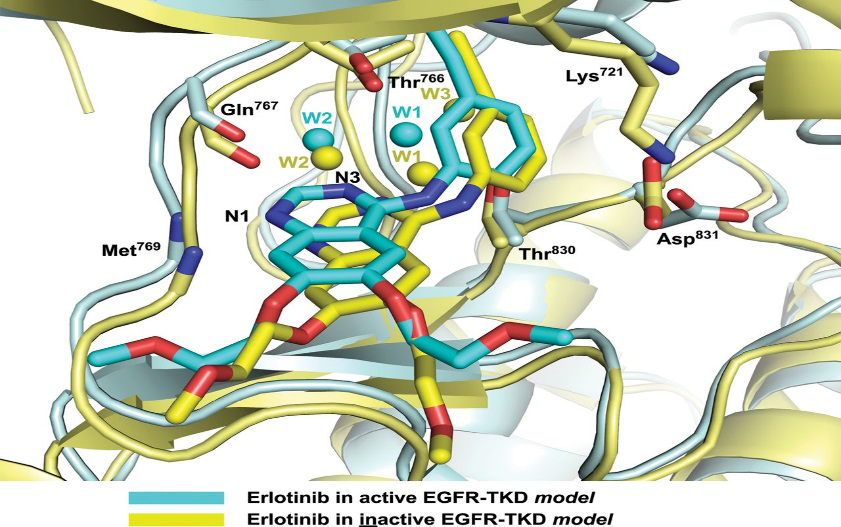 It is in this landscape that a new biotechnological start-up named Yihuo Bio steps in. The company is headquartered in USA, and is building in a manufacturing factory in China to produce en mass a new device that could revolutionize the lung cancer field as soon as the summer of 2016. They will make an official product presentation in the next ASCO conference this coming June in Chicago, USA.The company is run by Dr. Wei Liao, Dr. Liao, a happily married family man studied chemistry in the most prestigious Chinese university in Beijing. He followed his studies in China with post-graduate studies in medicine at UCLA. As he states, he has a clear vision of the future of his company. "The more people that can benefit from this new technology, the better," he earnestly states. "We own the patent but we do not mind sharing with anyone who has the same vision as us; we want to help as many people as possible," he adds. They plan to start producing in China and spread rapidly throughout the domestic market –the second-largest economy in the world. As soon as 2017, they plan to penetrate into other regions.The device is convenient in its usage but complex in its structure. It can detect most of the common lung cancer subtypes, non-small cell lung cancer through analysis of a simple body fluid. This process has been called a "liquid biopsy" and gives the same result as a regular biopsy without the invasive drawbacks. The advantages of this new lung cancer diagnosis method compared to previous ones are self-evident. Aside from it being non-invasive, it has proven inexpensive efficient.-It can diagnose most common lung cancer subtypes in as little as 30 minutes. In addition, tests have shown it presents a high detection accuracy rate, close to 99%.Regarding its structure-, it consists in the adaptation of a saliva-based EGFR mutation detection into an electrochemical platform integrating sensitive and specific multiplexing lab assay. The latter is named electric field-induced release and measurement (EFIRM). The combination of both structures and the final product name is eLB. This product adaptation has a significant meaning- to match Dr. Liao´s significant vision. Using an analogy-: he wants the platform to become the new "Apple Store" of gene mutation disease detection. The platform will be free. The idea is to be able to detect any disease able to be detected through gene mutation in the near future. The device would be comprised of numerous tests ticks. Each test tick can measure an EGFR mutation and is disposable. Its expected price is half' of current diagnosis methods.This product has two clear advantages related to its potential competitors. Nowadays, all other potential diagnosis methods are based on polymerase chain reaction (PCR) procedures. This has clear weaknesses- given that it requires a biopsy. It is thus considered an invasive diagnostic method. Furthermore, in order to detect a gene mutation, PCR requires advance knowledge of which DNA/RNA sequence you are targeting. Secondly, its accuracy detection is lower than eLB. PCR accuracy can also only be improved slightly by amplifying the sample. In contrast, eLB only amplifies the signal reducing time-consumption and costs. Overall, the PCR diagnostic method is costly at around US$450 in China and is especially time-consuming, taking between 10-14 days. By contrast, SABER dx is expected to cost around US$50 and takes no more than 30 minutes.The company is expected to start manufacturing its product in summer 2016. This new revolutionary product can be used in either hospitals or center labs. Unfortunately, for hospitals, the company is still working on the necessary administrative steps to begin commercializing its product. However, center labs, which determine their own regulations can being reaping the benefits of this new product as soon as it enters the market.
It is in this landscape that a new biotechnological start-up named Yihuo Bio steps in. The company is headquartered in USA, and is building in a manufacturing factory in China to produce en mass a new device that could revolutionize the lung cancer field as soon as the summer of 2016. They will make an official product presentation in the next ASCO conference this coming June in Chicago, USA.The company is run by Dr. Wei Liao, Dr. Liao, a happily married family man studied chemistry in the most prestigious Chinese university in Beijing. He followed his studies in China with post-graduate studies in medicine at UCLA. As he states, he has a clear vision of the future of his company. "The more people that can benefit from this new technology, the better," he earnestly states. "We own the patent but we do not mind sharing with anyone who has the same vision as us; we want to help as many people as possible," he adds. They plan to start producing in China and spread rapidly throughout the domestic market –the second-largest economy in the world. As soon as 2017, they plan to penetrate into other regions.The device is convenient in its usage but complex in its structure. It can detect most of the common lung cancer subtypes, non-small cell lung cancer through analysis of a simple body fluid. This process has been called a "liquid biopsy" and gives the same result as a regular biopsy without the invasive drawbacks. The advantages of this new lung cancer diagnosis method compared to previous ones are self-evident. Aside from it being non-invasive, it has proven inexpensive efficient.-It can diagnose most common lung cancer subtypes in as little as 30 minutes. In addition, tests have shown it presents a high detection accuracy rate, close to 99%.Regarding its structure-, it consists in the adaptation of a saliva-based EGFR mutation detection into an electrochemical platform integrating sensitive and specific multiplexing lab assay. The latter is named electric field-induced release and measurement (EFIRM). The combination of both structures and the final product name is eLB. This product adaptation has a significant meaning- to match Dr. Liao´s significant vision. Using an analogy-: he wants the platform to become the new "Apple Store" of gene mutation disease detection. The platform will be free. The idea is to be able to detect any disease able to be detected through gene mutation in the near future. The device would be comprised of numerous tests ticks. Each test tick can measure an EGFR mutation and is disposable. Its expected price is half' of current diagnosis methods.This product has two clear advantages related to its potential competitors. Nowadays, all other potential diagnosis methods are based on polymerase chain reaction (PCR) procedures. This has clear weaknesses- given that it requires a biopsy. It is thus considered an invasive diagnostic method. Furthermore, in order to detect a gene mutation, PCR requires advance knowledge of which DNA/RNA sequence you are targeting. Secondly, its accuracy detection is lower than eLB. PCR accuracy can also only be improved slightly by amplifying the sample. In contrast, eLB only amplifies the signal reducing time-consumption and costs. Overall, the PCR diagnostic method is costly at around US$450 in China and is especially time-consuming, taking between 10-14 days. By contrast, SABER dx is expected to cost around US$50 and takes no more than 30 minutes.The company is expected to start manufacturing its product in summer 2016. This new revolutionary product can be used in either hospitals or center labs. Unfortunately, for hospitals, the company is still working on the necessary administrative steps to begin commercializing its product. However, center labs, which determine their own regulations can being reaping the benefits of this new product as soon as it enters the market.
文|Jopevi 陈坤
责编|张楠
如果您想对接动脉网所报道的企业,请填写表单,我们的工作人员将尽快为您服务。